Clinical issues of Technegas use
VTE [venous thrombo-embolism] is an uncompromising predator that can gnaw at the very strands of life itself. Yet it develops slowly, usually unseen and unheralded, to burst upon clinical consciousness with frightening rapidity.
So wrote Henry W Gray as part of a dramatic personalised introduction to his excellent review ‘The Natural History of Venous Thrombo-embolism: Impact on Ventilation/Perfusion Scan Reporting’ published in Seminars in Nuclear Medicine vol XXXII, 159-172, 2002.
Technegas was the name coined in November 1984 to describe what we now know to be a specialised sub-set of nano-encapsulated carbon composites. Technegas is inhaled as the ventilation component of a radio-diagnostic test for Pulmonary Embolism (PE). Technegas, as produced in a purpose-built apparatus for lung ventilation work, consists of hexagonal flat crystals of Technetium metal cocooned in multiple layers of graphite sheets completely isolating the metal from the external environment. Each particle is from 5-30nm in cross-section and 3nm thick, and is suspended in an argon carrier gas as a consequence of its production. The discovery of Technegas was the outcome of an eight years search for the 'ideal' diagnostic ventilation agent to complement Technetium-labelled macro-aggregated albumin (MAA) in the combination V/Q radionuclide diagnostic examination for the differential diagnosis of PE or blood clots in the lung. A commercial apparatus was developed in conjunction with a small engineering firm in Sydney, Australia, and the first machines sold locally in 1986. Now over 2500 machines are in use in 51 countries where about 200,000 diagnostic examinations are performed each year. Well over 2,000,000 patients have been studied with Technegas since 1986 without a single report of adverse events attributable to the test itself logged by Regulatory authorities anywhere. A modified production technique to improve particle size uniformity and yield is available in a second generation machine and known as 'Technegas Plus'. This page covers the medical issues behind the search for what ultimately became Technegas. Specific applications other than PE, the detailed technical nature of Technegas, the history behind its discovery, etc are covered on pages so named.
The Diagnostic Difficulty of Pulmonary Embolism
Further illustration of the importance of an urgent correct diagnosis and therapy is given by Dr. Frank Broderick in a personal review for this site of a 47 year Physician practise:
“I had a dramatic introduction to pulmonary embolism in my first year as an intern in 1955. The 40 year old woman I was called to see felt uneasy in the chest. She denied chest pain or breathlessness but was sure that something was about to happen. Her right medial meniscus had been removed a week before. There were no abnormal findings apart from the healing knee. I had just finished reassuring her and was walking to the door when she sat up, gave a little cry and died.
At autopsy the cause of death was a large embolus straddling the bifurcation of the pulmonary artery. A small to medium sized embolus, perhaps of a day or two duration, was present in the left lung. Presumably it had given rise to her feeling of unease.
I commenced lung perfusion scanning in 1967 adding ventilation scanning in 1976 with a variety of agents and from 1986 with Technegas exclusively, performing about 10,000 studies in total. From 1966 to 1985 I combined Nuclear Medicine with internal medicine which gave me greater insight into patient outcomes and sometimes subsequent unmasking of underlying disease.
In the post operative and post fracture group, the smallest injury I can recall was of a 44 year old plumber who fractured a 2nd metatarsal. It was pretty painful and various measures were tried to immobilise the fracture site, including two weeks in a plaster cast. He had no chest pain but became quite breathless and I was asked to scan him one night. There was very little perfusion of the whole of the right lung and the left lower lobe. He was admitted to intensive care and commenced on a continuous heparin infusion. He died suddenly at breakfast next morning. Autopsy showed multiple emboli in both lungs with recent embolisation to the left upper lobe, the only significant area of perfused lung seen on his scan of the previous night. In this case, a relatively small final embolus administered the coup de gras because of an already severely compromised lung function.
In the 1970's and 80's I looked after considerable numbers of people with pulmonary emboli. Most seemed to fit into one of two categories; either small emboli that produced pleuritic pain, often severe but with little haemodynamic effect or large emboli presenting with breathlessness and features of acute pulmonary hypertension, right heart strain and sometimes frank right heart failure. The presence of associated lung infarction would add haemoptysis, fever and pleural effusion. These latter features meant one often had to exclude other causes of lung consolidation.
Small emboli are important because they are often precursors of larger more haemodynamically significant emboli. They commonly give pleuritic pain probably because they slide into the smaller branches of the pulmonary artery and come to rest in a sub-pleural location. They may also be important in their own right. If there are enough of them they may seriously compromise lung function or more rarely give rise to severe longstanding pulmonary hypertension”.
Pulmonary Embolism (PE) is currently listed as the third highest cause of death in hospitalised patients in the USA. A national survey published back in 1975, as summarised in the diagram below adapted from that survey, is still being quoted in support of the need to take care in ensuring the correct diagnosis is made. It is generally agreed that a clinical differential diagnosis is only accurate in about 60+% of cases, underlining the critical importance of a high quality, rapid, safe and easily performed screening test.
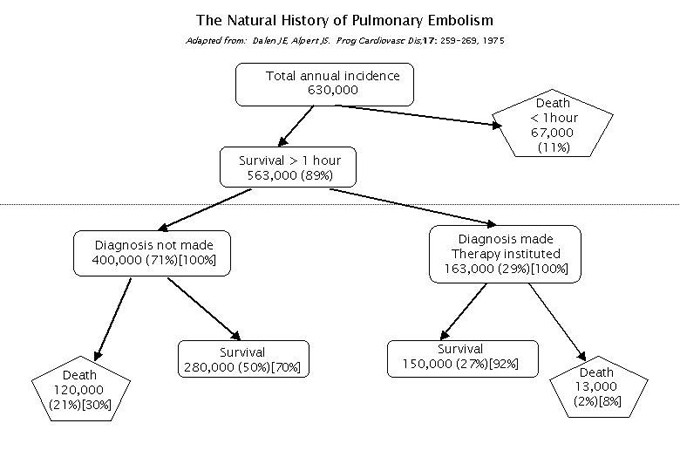
These statistics emerged at about the same time as the ventilation/perfusion (V/Q) procedure was evolving in Nuclear Medicine departments as a diagnostic tool for PE. Macro-aggregated albumin (MAA) labelled with 99mTc was, from the very start, recognised as the ideal perfusion agent. But there arose a plethora of ventilation agents, all of which were considerably inferior in terms of providing a true congruent image of airways distribution, to the MAA perfusion under routine clinical conditions. As a consequence, publication of numerous ‘algorithms’ for reporting the V/Q images left many referring Clinicians feeling they were not much better off than trusting in their own clinical judgement for a diagnosis.
PIOPED
Then came the publication of the results of PIOPED (Prospective Investigation of Pulmonary Embolism Diagnosis JAMA 1990: 263;2753-2759)a major USA-based multi-centre clinical trial that for the first time set out to put some quantisation into the V/Q image result data against the ‘gold standard’ of pulmonary angiography. There were subsequent publications reinterpreting the data, and ultimately a 'PIOPED II' in 2002 (Seminars in Nuclear Medicine volXXXII; 173-182; Gottschalk A et al.). In a sense PIOPED II reflected the anomalous position the USA Nuclear Medicine fraternity had found itself in for not having access to high quality ventilation agents, and the concomitant imaging techniques, largely because of regulatory inhibitions.
They were still locked in to >30 year old technique of single view planar images with 133Xe, or at best, 8 view planar DTPA aerosol studies as the ventilation (V) component of a V/Q study to be compared with the latest X-ray tomographic technology (MDCT). Meanwhile, in all other countries where advanced Nuclear Medicine is practised, very high quality tomographic V/Q is now demonstrated, through numerous peer reviewed publications (listed chronologically on this site) to, at worst, be equal in specificity and sensitivity to MDCT without the excessive radiation dose, or hazard from injecting x-ray contrast materials.
Indeed, at the 2005 European Nuclear Medicine Congress in Istanbul, at an invited presentation, Professor Carl Schümichen from Rostock in Germany noted "In the individual patient, both occlusive and non-occlusive emboli are present, otherwise a high sensitivity of scintigraphy could not be achieved. In a patient-by-patient analysis (PE yes or no), V/Q scanning is superior to multislice CT and sensitivity of scintigraphy is increased even more by SPECT." The full abstract of his talk can be found appended to this page.
SPECT Imaging
Much needed objectivity has now been brought to the SPECT story in a major report from Australia’s most experienced respiratory imaging group. By measuring V/Q ratios they have been able to demonstrate a high diagnostic accuracy in patients with suspected PE. Their objective analysis has the potential to reduce the number of non diagnostic scan results, and may be useful for quantifying V/Q mismatch in other pulmonary disorders. (Biblio ref# 236).
A new reference library facility is being assembled, based on clinical case studies, with the objective of helping Clinicians enhance their skills in this often difficult diagnostic arena. It is to be found at www.spectlung.com and visitors are urged to enhance its value by contributing one or more of their cases.
Image fusion and subtraction
Multi-slice computed tomographic angiography (CTA), an x-ray imaging modalitiy has displaced V/Q imaging for PE in some centers. But with the availability of Technegas tomographic ventilation to complement the perfusion studies and the use of the 'quotient' software concept, there is increasing recognition that the 'indeterminate' rate for V/Q is no worse than CTA. Ease of patient compliance alone puts the V/Q Nuclear Medicine modality well in front. Ultimately, it seems to come down to proper recognition that PE demands an urgent and correct diagnosis and treatment.
A Screening test for Pulmonary Embolism?
That so many different 'tests' for PE are extant after all these years of new and better medical diagnostic procedures, is proof enough that no universally recognised screening test exists; and that a final diagnosis is arrived at via various pathways, depending on clinical experience and technology availability inter alia.
Ideally any screening test should be non-invasive, or trivially so, for example a venous blood sample and applicable for all patients. But the more serious the consequences of a missed diagnosis will to a large extent raise the threshold of invasiveness tolerated for that diagnosis to be made. Hence the tendency to ignore ‘hidden’ invasiveness like radiation burden, by using computerised tomography pulmonary angiograms (CTPA) for seeking to differentiate PE.
The greatest single factor leading to this trend amounts to a perception that higher resolution is better because it will lead to more definitive diagnoses of smaller lesions. And coupled with that, is the lack of current high quality literature defining a practical threshold lesion size equally met by other less invasive technologies such as V/Q. The first report of a threshold lesion size of ≥ 0.5 segment by Howarth and his colleagues(1), was established through outcome studies on 924 patients who had V/Q as their primary investigation. It will hopefully stimulate replication and validation and should lead eventually to a sensible re-appraisal of the importance of this less invasive and more universally applicable technology for PE diagnosis.

Breath-hold transverse slice at level of hilum.
Note penetrations of the main bronchi.
Unlike any Radiology procedure, the Nuclear Medicine patient’s dose is committed at injection (or inhalation) of the radiotracer. Resolution for a given detector and processing system approaches the limiting value as the counts rise and in practice is a trade-off between tolerable imaging time linked in turn to patient or organ movement. This transverse slice from one of our research group in the mid 1990’s at about the level of the hilum, shows what is technically achievable with a volunteer, performing 64 breath-hold manoeuvres and imaged on a 30 year old single head GE Camera.
Radiological imaging, by contrast, demands more photons passing through the body in order to deliver better resolution. Hence the now oft-expressed concern particularly about breast dose in multi-slice CTPA.(2)
But if a lesion size threshold of clinical importance as described by Howarth (1) can be validated, then the need for spatial resolution beyond the limits of gamma cameras vanishes and V/Q can return to its rightful place as the pre-eminent screening test for PE, eliminating both the higher radiation burden and the significant number of patients (~15%) who cannot be imaged for reason of hazard from contrast injection or complete inability to breath-hold.
Mammography is a standard screening test for breast cancer delivering a non-trivial radiation burden, and it is instructive to compare as Parker did (2), for example, the dose received from that test with a CTPA. He finds that CTPA delivers a minimum of 20mGy breast dose compared with 3mGy for mammography. A combined V/Q procedure using 37MBq of Technegas followed by 150MBq of MAA delivers only 1.4mGy, or about half the mammography dose to the female breasts.
How significant is size for PE?
In principle, any PE reflects a dissecting thrombus somewhere in the venous circulation, and some have argued that inconsequential minor PE’s could arise routinely in the course of the body’s vascular maintenance activity. But what really does constitute that all-important warning ‘flag’ bringing the patient in and leading to anti-coagulant therapy? Howarth’s report (1) has given a sound basis from which to work, and its important to recognise that the 0.5 segment threshold is a cumulative figure that can be made up of multiple smaller elements. As noted in Harper’s Biochemistry(3), the initial clot formed in the complex cascade of thrombus production is rather weak, held together only by the non-covalent association of fibrin monomers. Only later do the covalent cross-links form, yielding a more stable fibrin clot. It may well be these fresh clots that further fragment in their turbulent passage through the right heart giving rise to the multiple small emboli often seen peripherally and accompanied most frequently by chest pain symptoms.
Härkönen (4), and his group from Finland have demonstrated that 14 of 43 cases (32%) who were CT negative demonstrated PE on V/Q SPECT. They were made up of 6 patients with a cumulative defect size equivalent of 1 segment, 4 with 1.5 segments and 4 with 2 segments equivalent defect volumes. These would all clearly qualify within the Howarth criterion of ≥ 0.5 segment minimum. More recently, European Guidelines have been published that endorse the concept of a 0.5 segment threshold (see bibliography ref #250, 251). Indeed, Professor Bajc and her team have taken the quantification of severity of PE to the point of managing patients, based on the percentage of lung affected, among other clinical factors. They have published their findings under the title 'Home treatment of patients with small to medium sized acute pulmonary embolism' (ref.# 304), finding from a cohort of 946 positive PE patients over five years, that their criteria can lead to safe out- patient therapy in 74% of cases.
Finding PE among other lung co-morbitities
One of the other significant findings from performing SPECT V/Q for PE specifically with Technegas as the ventilation agent, is that a differential diagnosis is most readily achieved. Increasingly, clinicians are finding this after using other inferior ventilation agents. (ref#299, 303).
Technegas as a sensitive marker for early COPD
A Swedish group has been developing an application using Technegas ventilation as a marker for early onset of COPD, more sensitive than conventional lung function tests.(ref #302,307).
References:
- Howarth DM, Booker JA, Voutris DD., 2006, Diagnosis of pulmonary embolus using ventilation/perfusion lung scintigraphy: more than 0.5 segment of ventilation/perfusion mismatch is sufficient. Int Med J 36; 281-288.
- Parker MS, Hui FK, et al., 2005, Female breast radiation exposure during CT pulmonary angiography. AJR 185:1228-1233.
- Murray RK, Granner DK et al (Eds)., 1993 Harper’s Biochemistry 23rd ed; p681, Publ. Prentice-Hall.
- Härkönen RA et al. Detection of pulmonary embolism by ventilation/perfusion SPECT in cases where helical CT was non diagnostic. Poster, WFNMB 2006 Seoul, Korea.











