Unveiling the role of extracellular vesicles in Alzheimer's disease
Few things are as heart-wrenching as watching our loved ones lose themselves to dementia, a syndrome impairing cognitive functioning.
With Alzheimer's disease being the most common form of dementia, the global impact of this condition is staggering, burdening over 55 million people worldwide.
Despite the tremendous economic and emotional burdens it imposes, Alzheimer's disease remains incurable, as scientists are still unravelling the puzzle of its development.
But now, neuroscientists at the John Curtin School of Medical Research (JCSMR) are embarking on a research project that could change the game.
They are investigating a group of tiny, lipid-bound particles called extracellular vesicles (EVs), which could potentially hold clues as the "messengers" in the progression of Alzheimer's disease.
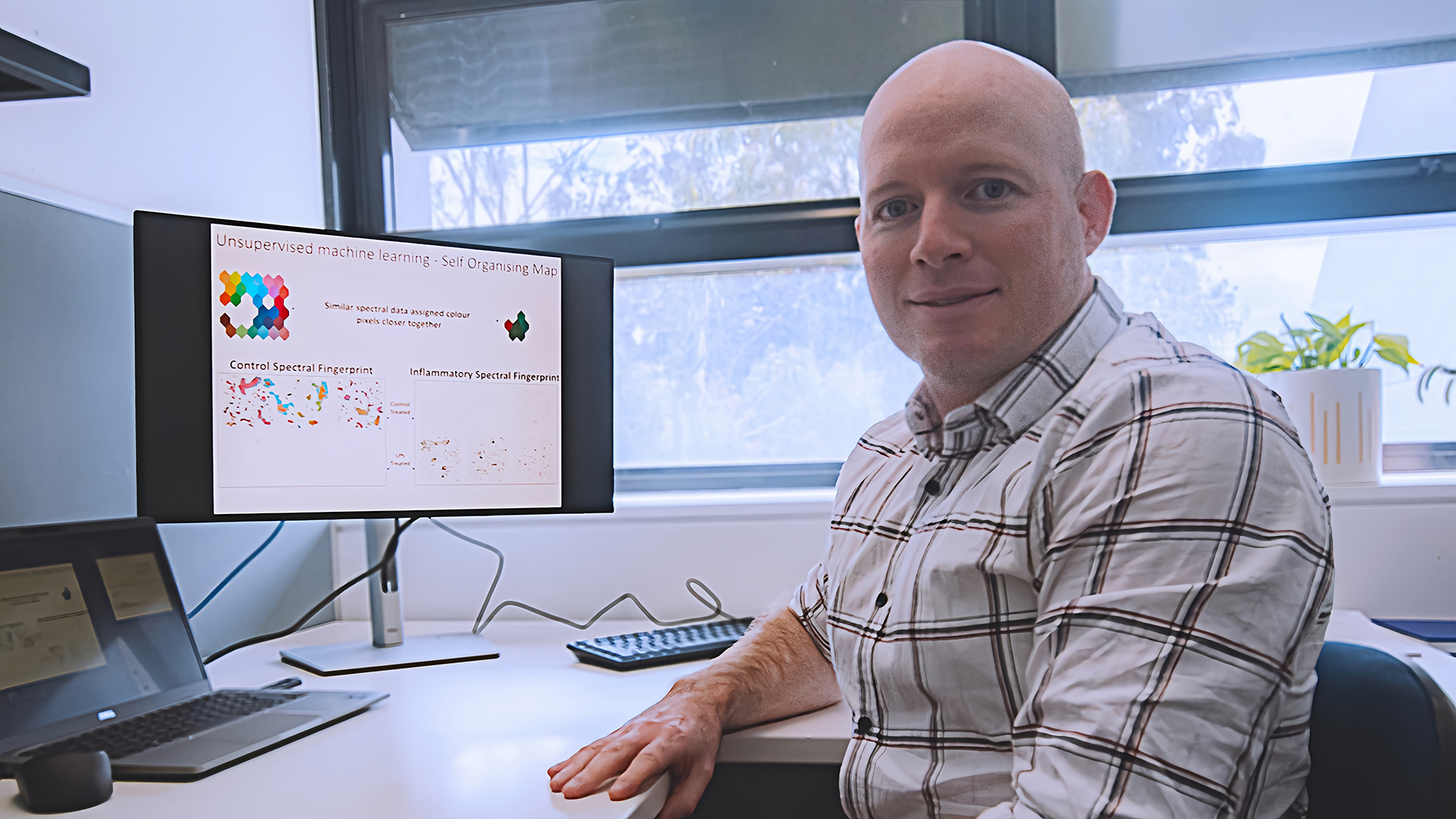
"Imagine EVs as microscopic packages of messages that cells use to communicate with each other," explained Dr Jereme Spiers, a Postdoctoral Research Fellow at the Natoli Group at JCSMR.
Not all messages they deliver, however, are accurate and functioning.
"EVs are associated with neuroinflammation, a major contributor to Alzheimer's disease," said Dr Spiers, "It has been hypothesised that damaged brain proteins and other biomolecules—pathogenic messages—may be packaged into these tiny vesicles and drive the progression of Alzheimer's disease in the brain."
Previous evidence in animal models has suggested reductions in EV biogenesis help delay the progression of the disease.
However, due to limitations in technology, little is known about which proteins EVs carry and which types of brain cells release these harmful vesicles.
This is where the cutting-edge techniques and artificial intelligence employed by Dr Spiers and his colleagues come into play.
They aim to acquire and compare the entire protein content of EVs sent from microglial cells of healthy and Alzheimer's disease brain tissue.
These "proteomic fingerprints", as the researchers put it, will then help identify specific types of brain cells that release harmful vesicles associated with neuroinflammation in Alzheimer's disease.
The significance of this research is not lost on the communities. Dr Spiers' project, 'Neuroinflammatory profile of microglial extracellular vesicles in Alzheimer's disease', has recently been awarded the Royce Simmons Foundation Mid-Career Research Fellowship by the Dementia Australia Research Foundation, highlighting its potential impact.
"Understanding how cells communicate inflammation in the brain is critical in combating the progression of neurodegenerative diseases," emphasised Dr Spiers.
With every three seconds marking a new diagnosis of dementia, this research offers hope for a better understanding of Alzheimer's disease and the possibility of developing new therapeutic targets.
By unlocking the secrets of EVs and their role in the pathogenic spread of Alzheimer's disease, this project could pave the way for innovative treatments and bring us closer to finding a cure for this devastating condition.
